Empirical Formula of Vitamin C
F drying the pellets in a high-temperature oven at about 120 C to a moisture content suitable for. These empirical formula of vitamin c are some of the best recommendations for you in this field.
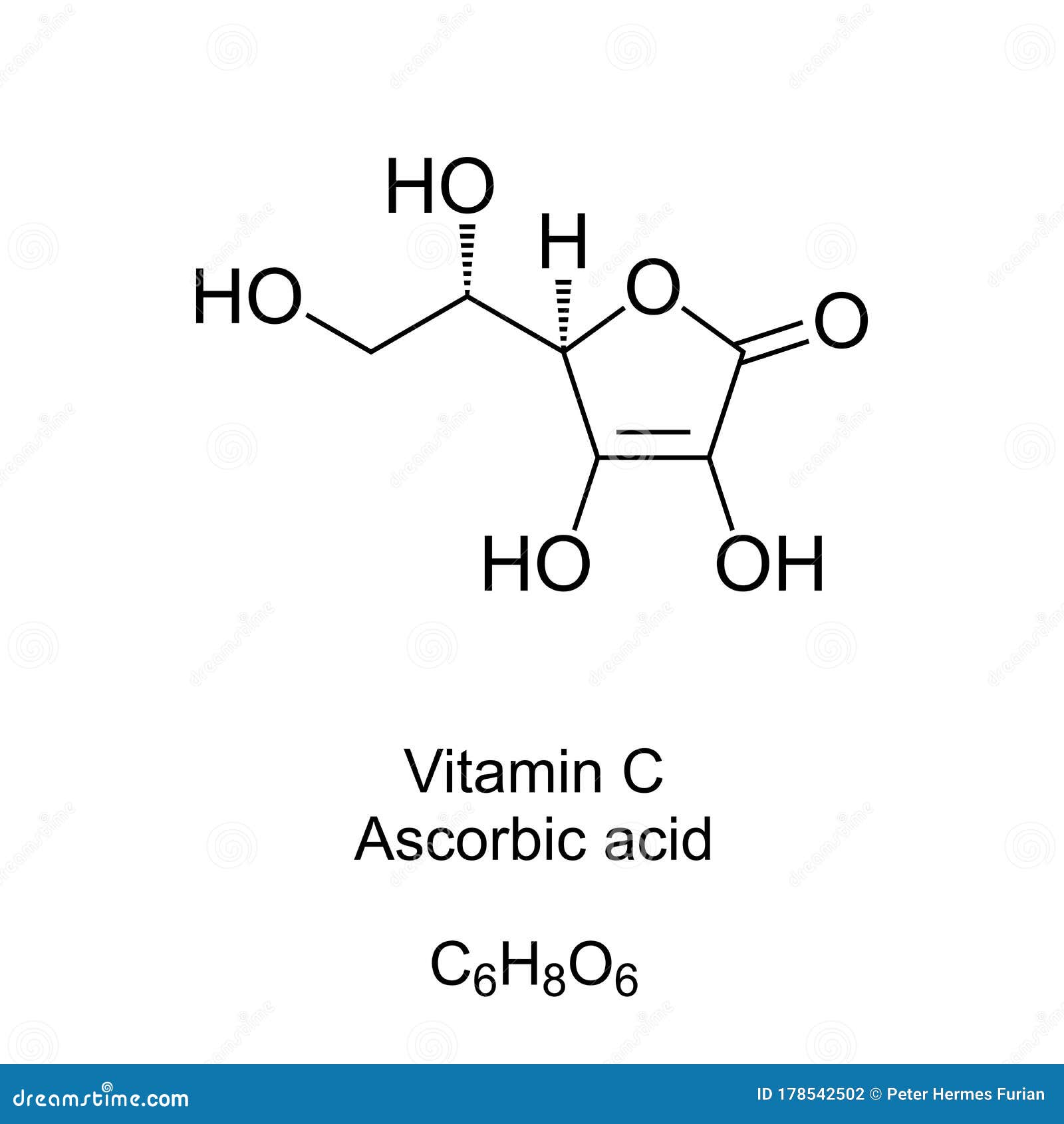
Download Vitamin C Ascorbic Acid Skeletal Formula And Molecular Structure Stock Vector Illustration Of Organic Chemical 178542502
Combustion of 02000 g of vitamin C gives 02998 g ceCO2 and 0819 g of water.
. C 3 H 4 O 3 2 Molecular formula. Empirical formula is the simplest chemical formula which depicts the whole number of atoms of each element present in the compound. From this formula we can say.
What is the empirical formula of vitamin CMy workI calculated moles of products and found the mole ratio but its not coming to be a whole number. What is the empirical formula of vitamin C. Ad Save on doctor recommended supplements herbs and nutritional formulas at Vitacost.
C H 2. Each day a persons diet should include a source of vitamin C such as orange juice. C 6 H 8 O 6.
If we are given the molecular mass of the compound we can find its molecular formula also. What is the empirical formula of vitamin. Shop save with Vitacost.
Answer 1 of 4. What is the empirical formula of vitamin C. Vitamin c is known chemically by the name ascorbic acid determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458 hydrogen and 5450.
Vitamin C also known as ascorbic acid is water soluble and cannot be produced by the human body. Combustion of 02000 g of vitamin C. C H 2 O.
So from that we calculate the molecular mass by performing the calculationmultiplying with individual. Get fast free shipping to your area on orders 49. You are in RIGHT PLACE.
6 2 3. Ad Find Deals on empirical vitamin c in Nutrition on Amazon. Therefore the empirical formula of the vitamin C is found to be C 3 H 4 O 3.
The empirical formula of vitamin C is C3H4O3. What is the empirical formula of vitamin C. 6 2 3.
8 2 4. Ascorbie acid vitamin C a white crystalline solid that is present in fruits and vegetables curves scury and may help. Which we list out some of best.
If you divide all three numbers by 2 you will get. The empirical formula is C 62 H 89 CoN 13 O 15 P and its structural formula is. For example vitamin C contains 4092 carbon 458 hydrogen and 545 oxygen by massIts empirical formula can be calculated by the following steps.
Simplest or empirical formula. I calculated moles of products. Calculating the empirical and molecular formulae of Vitamin C from its percentage compositionHelpful for NCEA Level 2 Chemistry.
C 6 H 8 O 6 or HC 6 H 7 O 6. The molecular formula of Vitamin. If we multiply all the subscripts in the empirical formula by 2 then our molecular formula will be.
Since you cannot longer divide by an integer to get a smaller whole number ratio the. This page reaches your expectation.
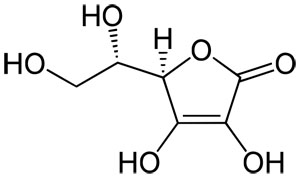
The Vitamin C Molecule Antioxidant Properties
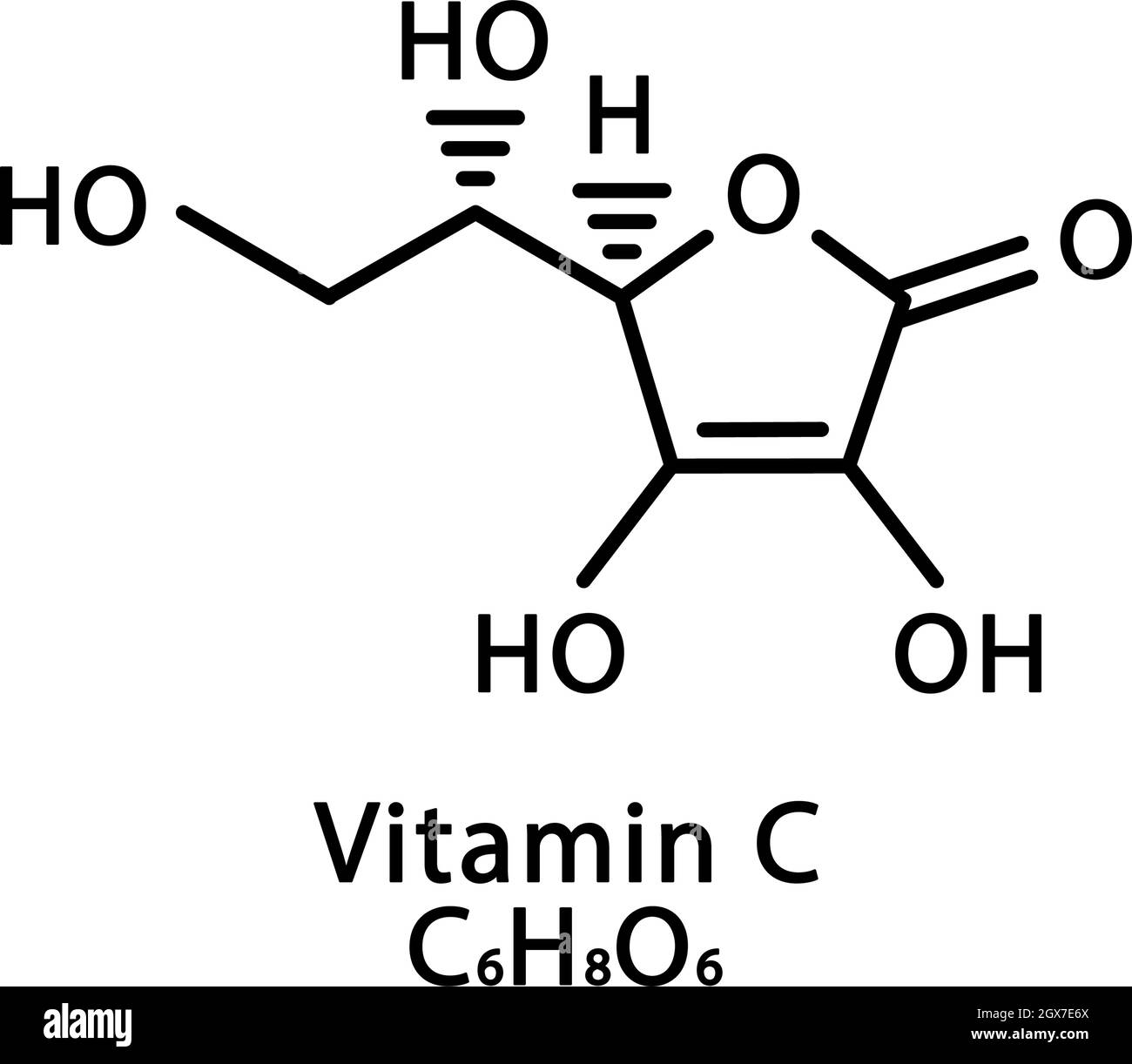
Vitamin C Ascorbic Acid Molecular Structure Vitamin C Ascorbic Acid Skeletal Chemical Formula Chemical Molecular Formulas Stock Vector Image Art Alamy
No comments for "Empirical Formula of Vitamin C"
Post a Comment